Onkogenetics
Tumor Mutation Burden
What is Molecular Profiling?
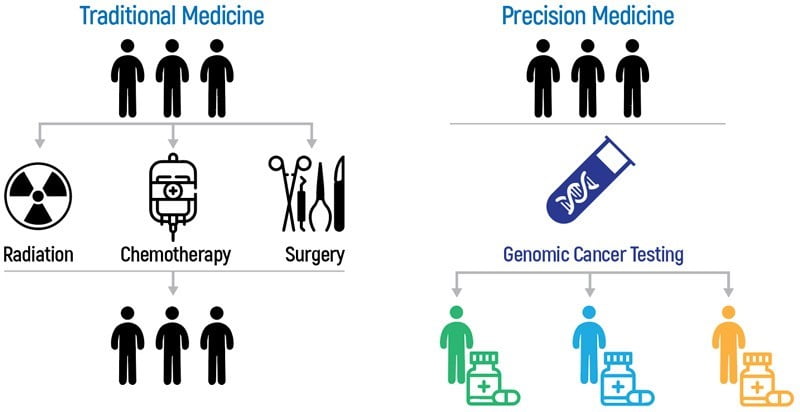
Detection of cancer-related biomarkers has become a fundamental step for a personalized treatment approach in cancer patients. The “OnkoGenetics™ Molecular Profiling” panel designed for this purpose offers a comprehensive genomic profiling analysis of formalin-fixed and paraffin-embedded (FFPE) or fresh tumor tissue samples. “OnkoGenetics™ Molecular Profiling” is used to identify DNA variants in different tumor types and also to detect the immunotherapy biomarkers Tumor Mutation Burden (TMY) and Microsatellite Instability (MSI) . Thanks to the use of next-generation sequencing (NGS) technology, this comprehensive test panel, which allows simultaneous screening of variants in 486 genes , can provide fast and comprehensive results even when the sample amount is low. With the data obtained as a result of OnkoGenetics™ Molecular Profiling and TMY panel:
- Targeted therapy
- Personalized treatment
- Evaluation of smart drug/immunotherapy options is possible
Why OnkoGenetics™ Molecular Profiling?
- Identification of germline mutations with Molecular Profiling from blood and calculation of a true TMY
- Detection of DNA variants, Microsatellite Instability (MSI) and Tumor Mutation Load (TMY) in 486 genes
- Identifying personalized treatment options
- Possibility of avoiding possible side effects of chemotherapy
- Results in a short time (15-21 working days)
- High sensitivity and reading depth (Use of Unique Molecular Index (UMI))
- Access to over 10 million biomedical findings and over 1 million unpublished variant-phenotype associations through the use of QIAGEN Bioinformatics solutions.
What is Tumor Mutation Burden (TMY)?
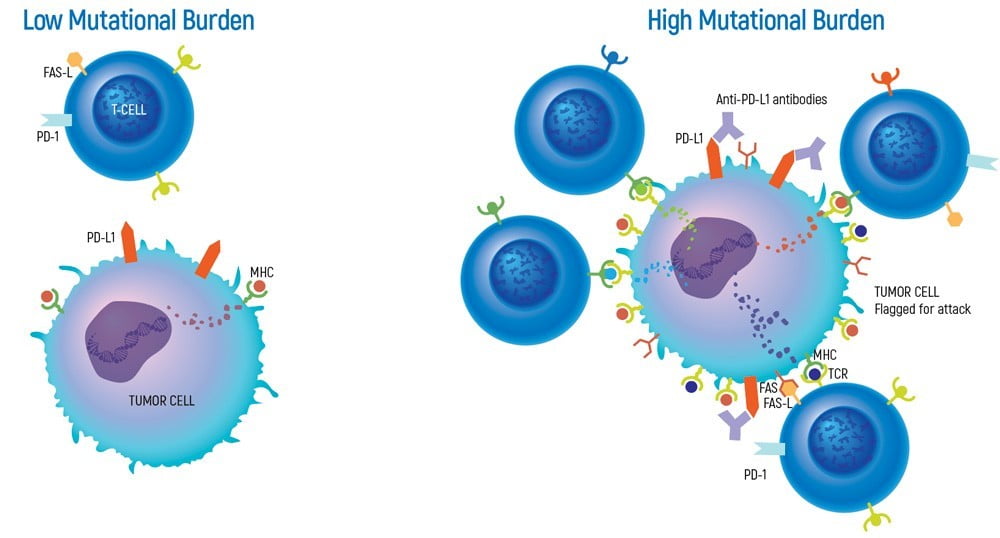
Tumor Mutation Burden is a new quantitative biomarker that can help predict responses to certain cancer immunotherapies. This value represents the total number of mutations per coding region of a tumor genome. By measuring the amount of somatic mutations in a tumor with TMY, the burden of neoantigens is approximated, thus serving as a marker for the likelihood of the patient benefiting from immunotherapy.
OnkoGenetiksTM Tumor Mutational Burden analysis reports.
- Presented in an easily interpretable format in order to provide the highest possible clinical benefit.
- Your physician can have access to your tumor profiling information in a clear and comprehensible manner.
- A turnaround time of 15-21 days helps you to find the answers you seek as fast as possible.
Liquid Biopsy
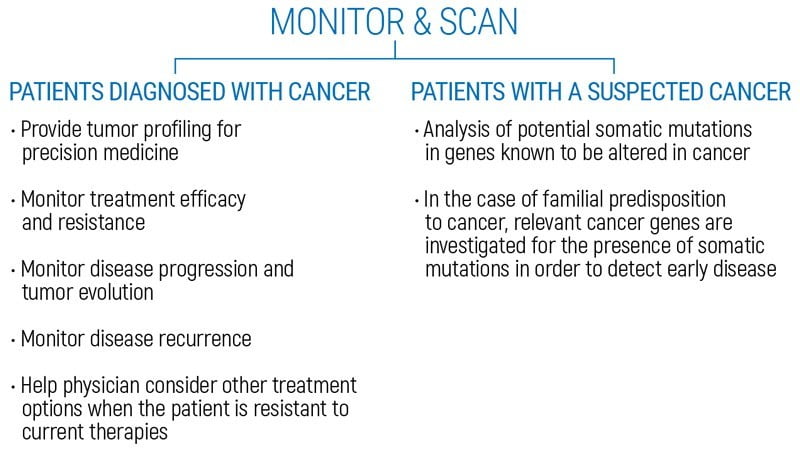
OnkoGenetiks Liquid Biopsy analyzes the free-floating tumor DNA (ctDNA) in a blood sample, enabling the detection of somatic mutations. “Liquid Biopsy” is a safe, highly sensitive and cost-effective method that can analyze free-circulating tumor DNA (ctDNA) obtained from the blood of people diagnosed with cancer or at risk of cancer. It can be used to detect cancer at an early stage, to help plan treatment, to determine how well a treatment has been working, or to see if the cancer has recurred. The OnkoGenetiks™ Liquid Biopsy test has been specially designed for people who have been diagnosed with cancer or are at risk for cancer, to detect somatic mutations in the DNA of free-floating tumor DNA (ctDNA) for the early diagnosis or follow-up of cancer.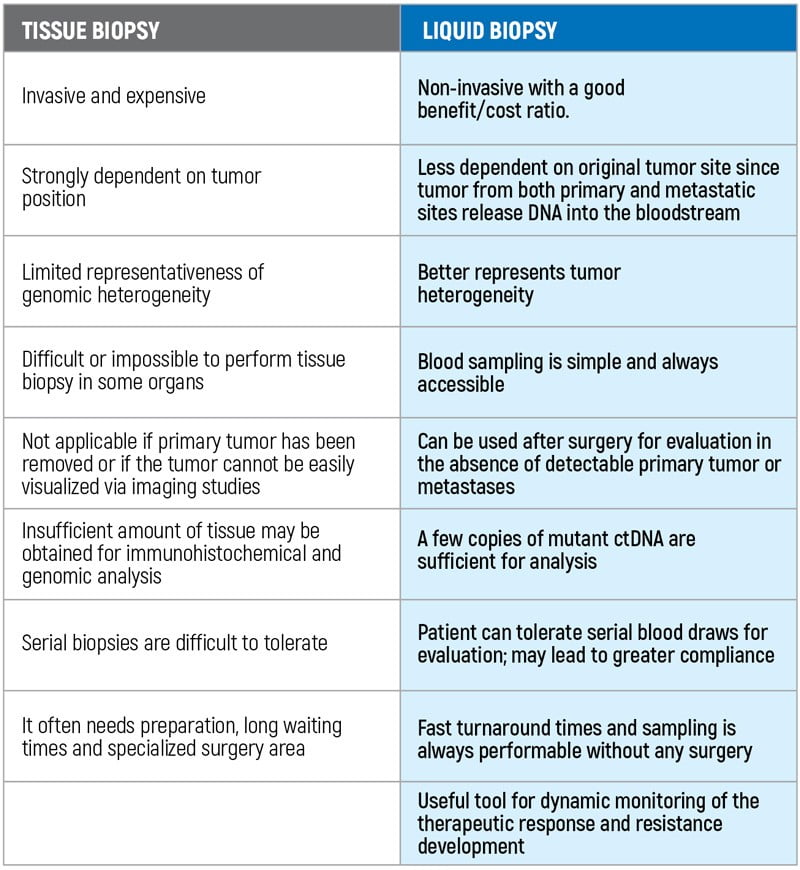
General workflow of OnkoGenetics™ Liquid Biopsy Analysis:
As the first step of the test procedure, ctDNA isolation is performed from the blood collected in PAXgene Blood ccfDNA tubes. After library creation and enrichment of target DNA regions with the QIAseq Targeted DNA Panel, Next Generation Sequencing (NGS) is performed on the Illumina NextSeq 550Dx sequencing platform. Variants detected after analysis of raw sequence data are interpreted using QCI™ (QIAGEN Clinical Insight) or IVA (Ingenuity®Variant Analysis) software. OnkoGenetiks Liquid panel contents have been designed by Genetiks experts.